Software company offers MoCRA compliance support via digitization

California-based software company Specright is addressing a key element of the impending implementation of MoCRA legislation with its patented cloud-based Specification Management software. The technology, which can assist manufacturers and suppliers to the cosmetics and personal care product industries to better achieve compliance with product listing data requirements, can be an asset to those companies looking to standardize their product and quality data through digitization.
To learn more about how Specification Management software like Specright’s can support manufacturing and supply companies to the cosmetics and personal care product industries transition to MoCRA compliance, the importance of considering digitization of crucial company data to adhering to MoCRA regulations, and recommendations for companies in the cosmetic and personal care product manufacturing and supply industries looking to start the process of adhering to the FDA’s MoCRA legislation, we spoke with Tom Preston, VP of Business Development at Specright for his insights.
CDU: How can Specright help cosmetics and personal beauty care companies comply with the impending MoCRA regulations?
Tom Preston (TP): A key element of MoCRA is “modernization” – and in many cases this means companies must digitize. Many currently rely on static and outdated processes to manage spec-level data and need to make the shift to digitizing their product, package, and processing information to better comply with the impending MoCRA regulations.
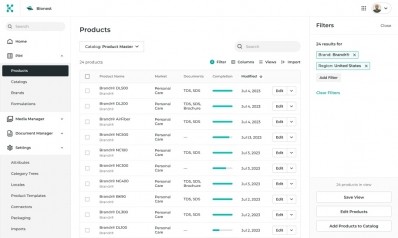
Specright’s patented Specification Data Management (SDM) platform allows companies to centralize and standardize product, packaging, and quality data, which in turn enables greater collaboration with customers, suppliers, and co-manufacturing partners.
On this searchable platform that provides real-time reports and dashboards, manufacturers and suppliers can manage all important files, ingredient listings, formula specifications, facility information, and critical data documentation in a digital, single source of truth.
Specright’s platform allows businesses to modernize how they are navigating and accessing these critical pieces of data to provide increased visibility, safety, efficiency, and sustainability when developing products. The platform also helps companies collaborate and share data with suppliers, track event reporting, and identify allergens and label requirements.
CDU: What are some recommendations for companies to start implementing now to ensure they are in compliance with MoCRA regulations by the time the regulations are begin implementation at the end of this year?
TP: Starting now, companies should evaluate the implications that this new legislation has on their business, identify any of the compliance requirements that will impact their company and develop a strategy, along with identifying the necessary tools, for better management and reporting of their product and packaging information.
Companies should also consider conducting an internal gap analysis to comprehend how the current processes need to be updated to comply with MoCRA. Updating a company’s standard operating procedures (SOPs) within the next few months is also a great first step in ensuring that a company is able to pivot to comply with the new requirements such as adverse event reporting, record keeping and facility registration.
CDU: What are some static processes that will hold a cosmetics or personal beauty care product manufacturer or supplier from being in compliance with MoCRA regulations?
TP: Static processes that require manual data assembly and do not have connected information will prohibit brand owners and suppliers from achieving compliance with MoCRA regulations. Some common examples of this are manual quality reporting, lack of internal systems for specification management and document management, and the use of outdated record sharing like emails and spreadsheets.
To best comply with these regulations, companies will want to establish a digital, single source of truth for all specification data that can be quickly updated and easily accessed by the manufacturer and its suppliers.
CDU: Can you explain the importance of digitizing critical product and packaging data for cosmetics and personal beauty care product manufacturers and suppliers in accordance with MoCRA specifications?
TP: By making the shift to digitizing one’s critical product and packaging data, manufacturers and suppliers are able to monitor for MoCRA compliance in real time. Instead of having the data accessible through various static channels, all data can be in one digital location that allows these companies to streamline reporting and benchmarking, and prioritize their time in other areas of the business.
CDU: Are there any challenges to MoCRA compliance that cosmetics and personal beauty care product manufacturers and suppliers should be aware of?
TP: Cosmetics and personal beauty care product manufacturers and suppliers should understand that it is critical to manage one’s data dynamically. Retrieving data from disparate systems is often difficult and by the time all of the data is properly gathered it is often outdated, leading to a delay in a company’s compliance with the new regulations.
The date of the FDA enforcement is set for Dec. 29, 2023 which is not that far away. Getting a head start on this now will allow a company to avoid any fines if not compliant with the new legislation.
CDU: Anything else to add?
TP: It’s important that companies not view this new MoCRA legislation as a penalty, but as the government’s way of standardizing how cosmetics companies maintain safe products for everyone. MoCRA regulations ensure that manufacturers and supplies are making safe, effective products – but modernizing through tools lite Specification Data Management platforms will also enable them to increase speed to market and sustainability tracking.




![Chinese study highlights mental health challenges in atopic dermatitis, emphasising holistic patient care. [Getty Images]](https://www.cosmeticsdesign-europe.com/var/wrbm_gb_food_pharma/storage/images/_aliases/wrbm_tiny/publications/cosmetics/cosmeticsdesign-asia.com/headlines/formulation-science/chinese-research-linking-atopic-dermatitis-to-mental-health-underscores-need-for-holistic-care/17040623-1-eng-GB/Chinese-research-linking-atopic-dermatitis-to-mental-health-underscores-need-for-holistic-care.jpg)





